By Jeremy Fregene
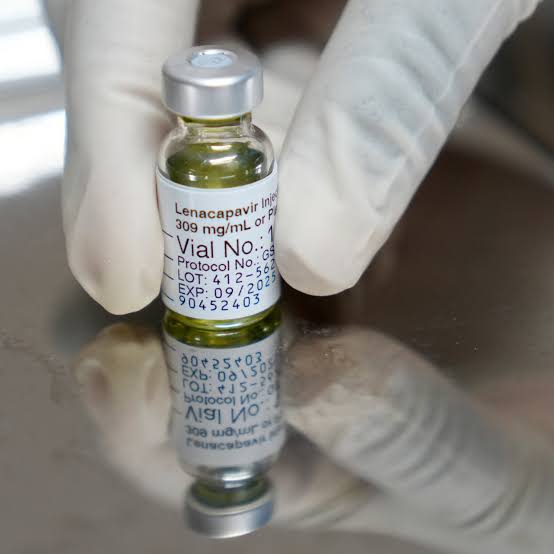
A major breakthrough in HIV prevention has been achieved, as the World Health Organisation (WHO) has endorsed lenacapavir (LEN), a twice-yearly injectable drug that provides near-total protection against HIV.
The landmark announcement was made on Monday 14 July, 2025, during the 13th International AIDS Society (IAS 2025) Conference on HIV Science, taking place in Kigali, Rwanda.
Described as a long-acting antiretroviral alternative to the daily pill regimen, lenacapavir represents a turning point in the global fight against HIV, particularly for vulnerable populations with limited access to consistent healthcare.
READ ALSO: Stakeholders Brainstorm on CRGIS Project, Emphasizing Inclusivity and User-Friendliness
WHO Director-General Dr. Tedros Adhanom Ghebreyesus hailed the development, calling LEN “the next best thing” in the absence of an effective vaccine to end the four-decade-old epidemic.
“Lenacapavir has been shown in trials to prevent almost all HIV infections among at-risk populations,” Dr. Tedros said in a statement released at the conference.
The drug works by blocking the HIV virus from replicating in the body. Unlike traditional antiretroviral therapies that must be taken daily, lenacapavir is administered just twice a year, offering both convenience and consistency.
WHO emphasized the urgent need for immediate global rollout of the medication and urged countries to integrate it into existing national HIV prevention programs without delay.
The agency called for equitable access through a variety of channels, pharmacies, community clinics, mobile health centres, and online consultations, to reach high-risk groups quickly and effectively.
To further improve prevention efforts, WHO also recommended the widespread use of rapid HIV testing, describing it as more practical and cost-effective than complex laboratory-based diagnostics.
The new guidance comes amid faltering global progress on HIV prevention, especially among key populations that continue to bear a disproportionate burden of the disease.
According to WHO data, 1.3 million people were newly infected with HIV in 2024. Sex workers, men who have sex with men, transgender individuals, people who inject drugs, incarcerated persons, and adolescents remain the most affected.
Currently, lenacapavir is largely confined to clinical trials and pilot studies, but WHO’s endorsement is expected to accelerate regulatory approvals and policy adoption across countries.
This recommendation aligns with the U.S. Food and Drug Administration’s (FDA) approval of lenacapavir in June, which deemed it safe and highly effective as a pre-exposure prophylaxis (PrEP) option.
In addition to lenacapavir, WHO reaffirmed support for other preventive tools such as daily oral PrEP, the injectable cabotegravir (given every two months), and the dapivirine vaginal ring used by women in high-risk settings.
However, experts say lenacapavir’s biannual dosing schedule and high efficacy could make it a preferred choice for those who struggle with daily medication or have irregular access to healthcare.
The call for urgent adoption also comes at a time of declining global funding for HIV/AIDS programs, including major cuts to the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR), which has been a cornerstone of HIV control efforts since 2003.
“We have the tools and the knowledge to end AIDS as a public health threat,” said Dr. Meg Doherty, Director of WHO’s Global HIV, Hepatitis, and STI Programmes.
“What we need now is bold implementation of these recommendations, grounded in equity and powered by communities most affected by HIV,” she added.
She warned that delays in adopting new technologies like lenacapavir could reverse the fragile gains made in curbing HIV transmission, especially in regions where stigma, discrimination, and poverty persist.
By the end of 2024, an estimated 40.8 million people were living with HIV globally, with about 65 percent of them residing in Africa, highlighting the continent’s urgent need for long-acting treatment options.
During the same period, 630,000 people died from HIV-related causes, a sobering reminder of the virus’s enduring toll despite advances in therapy and prevention.
On a brighter note, the number of people on antiretroviral treatment continued to rise, reaching 31.6 million in 2024, up from 30.3 million in the previous year, according to the WHO.
Public health experts say widespread use of lenacapavir could further reduce infections and deaths, especially among those with limited access to daily pills or who face barriers such as mobility, stigma, or forgetfulness.
WHO said it is working with governments, donors, civil society organisations, and pharmaceutical manufacturers to ensure affordability and distribution of lenacapavir across all income settings.
As the global health community gathers in Kigali to reassess the path toward ending AIDS, the message from WHO is clear: the tools to stop HIV exist—and lenacapavir could be a game-changer if the world acts quickly and inclusively.